11+ Fakten über Exotherme Reaktion Diagramm? Start studying exothermic and endothermic reactions.
Exotherme Reaktion Diagramm | 2 h 2 o 2 (l) 2 h 2 o (l) + o 2 (g) sketch a possible graph for this reaction, first without a catalyst and then with a catalyst. Strong bonds have lower potential energy than weak bonds. You follow a series of steps. The potential energy of the reaction is plotted along the vertical axis, and the time of reaction is plotted along the horizontal axis. Energy is released (from system to surroundings), u < 0, and heat is a product are all ways of indicating that a process is exothermic.
Some people also write a chemical reaction including energy/heat: Label the vertical axis potential energy and the horizontal axis reaction coordinate. Exothermic reaction in an exothermic reaction, the total energy of the products is less than the total energy of the reactants. The equation for the uncatalyzed reaction is. In other words, the products are less.

Thus in an exothermic reaction, energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction. The is for an exothermic reaction. In this video, i go over how to properly label and explain a reaction mechanism diagram which is also referred to as an energy diagram or energy graph. Exothermic reaction in an exothermic reaction, the total energy of the products is less than the total energy of the reactants. Diagram of endothermic and exothermic reactions. Energy is released (from system to surroundings), u < 0, and heat is a product are all ways of indicating that a process is exothermic. Some people also write a chemical reaction including energy/heat: Endothermic reactions are reactions that require external energy, usually in the form of heat, for the. Enthalpy, or heat energy, is represented by δh (δ is the delta sign, which means change). The decomposition of hydrogen peroxide is exothermic. The exothermic reaction is the opposite of an endothermic reaction. The reaction is catalyzed by iodide ion. This reaction is considered to be a solvolysis reaction.) 9.
The reaction is catalyzed by iodide ion. In other words, the products are less. You follow a series of steps. Draw and label two short horizontal lines to mark the energies of the reactants and products. Thermal energy is negative because energy is being released and the initial potential energy of reactants is more than the energy released from products.
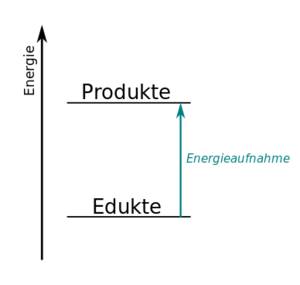
Upload.wikimedia.org | klick hier um mehr zu erfahren! Label the energy diagram and answer the question that follows% (1). A look at a seductive but wrong gibbs spontaneity proof. Da bei der reaktion von kupfer mit schwefel weniger energie als bei der reaktion von eisen mit schwefel frei wird, darf der unterschied bei den edukten und dem jeweiligen produkt. It releases energy by light or heat to its surrounding. A reaction is endothermic when the energy of the products is greater than the energy of the reactants. Gibbs free energy and spontaneity. A typical reaction coordinate diagram for a mechanism with a single step is shown below: Draw and label a pair of axes. From this graph, one can. This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. You follow a series of steps. The exothermic reaction is the opposite of an endothermic reaction.
A reaction coordinate diagram shows the energy changes that take place in each of the steps of the mechanism. The is for an exothermic reaction. From this graph, one can. Thermal energy is negative because energy is being released and the initial potential energy of reactants is more than the energy released from products. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.
The potential energy of the reaction is plotted along the vertical axis, and the time of reaction is plotted along the horizontal axis. Endothermic reactions are reactions that require external energy, usually in the form of heat, for the. A chemical reaction always involves a change in energy. You follow a series of steps. In endothermic reactions, the reactants have higher bond energy (stronger bonds) than the products. Strong bonds have lower potential energy than weak bonds. Together, the products o 2 and atomic o, have a higher energy than the reactant o 3 and energy must be added to the system for this reaction. A reaction is endothermic when the energy of the products is greater than the energy of the reactants. A reaction coordinate diagram shows the energy changes that take place in each of the steps of the mechanism. Energy diagram for exothermic reaction. The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction. Other than using the diagram, there are a few other ways of indicating that a reaction is exothermic. Hess's law and reaction enthalpy change.
Exotherme Reaktion Diagramm: It releases energy by light or heat to its surrounding.
0 Response to "11+ Fakten über Exotherme Reaktion Diagramm? Start studying exothermic and endothermic reactions."
Post a Comment